Antimicrobial Stewardship
The use of information technology to support antimicrobial stewardship
Key points
- The clinical context within which prescribers must make decisions can be complex. Infections can be difficult to treat, and various factors need to be considered. Decision support tools can assist prescribers by presenting useful information at the point of care.
- Computerised decision support systems (CDSS) can support AMS programs by providing mechanisms for key stewardship processes, including approvals and review. CDSS can also assist with surveillance activities.
- The effectiveness of CDSS for antibiotic stewardship has been researched and affirmed by key professional bodies.
- CDSS can include mobile applications, approval systems, medication management systems, infection prevention and surveillance systems (and other back-end systems), and advanced systems.
- Several factors can influence whether a CDSS is implemented successfully, including organisational readiness and system functionality.
- There are barriers to successful implementation, including cost and infrastructural capacity. Importantly, clinicians need to be involved in every step of the implementation.
- References for the information on this page can be found in the book chapter here.
Overview
Information technology (IT) is an important component of AMS programs and can be harnessed to support the AMS team as well as to support prescribers. At a minimum, AMS programs should have access to data from pharmacy dispensing or prescribing systems, and the microbiology laboratory system (if available). In this section, we will discuss opportunities and methods for providing decision support for antimicrobial prescribing in hospitals. The state of IT systems in LMIC hospitals may not support a sophisticated integrated program, and so mobile applications are likely to play an important role.
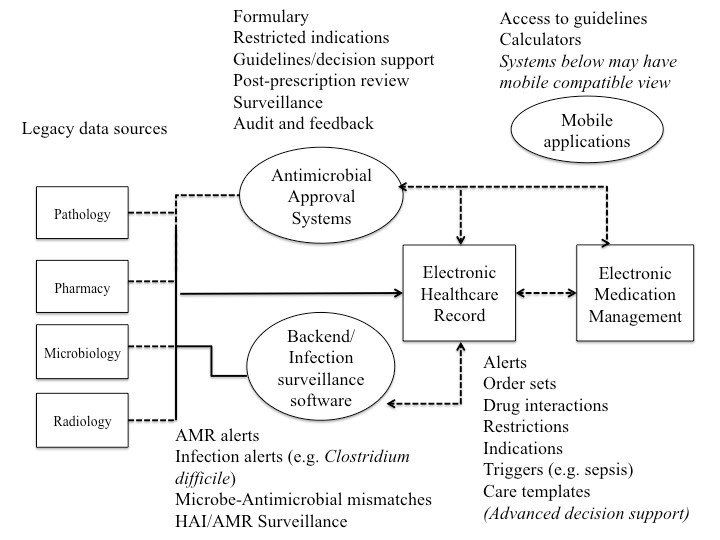
The figure below shows an overview of IT systems and potential opportunities for the provision of decision support.
Large amounts of information about patients, likely pathogens, treatment options, and potential drug interactions, contraindications and adverse reactions need to be processed by clinicians at the point of care. Prescribing decisions can be enhanced using clinical guidelines and algorithms, formulary restrictions, and computerised rules and alerts for antimicrobial therapies.
The AMS team, whose role it is to optimise the quality of care by monitoring and reviewing antimicrobial treatments, can benefit from IT systems that support post-prescription review, audit, reporting and feedback.
Studies have demonstrated that these systems can:
- increase appropriateness of prescribing;
- reduce medication and dosing errors;
- reduce the burden of healthcare-associated infections;
- minimise costs and antimicrobial expenditure per patient or increase savings for the hospital;
- reduce total medical expenditure; and
- reduce length of stay.
Types of antimicrobial decision support systems
Currently, the most commonly used computerised decision support systems (CDSS) for AMS are:
- electronic guidelines and mobile applications;
- electronic antimicrobial approval systems;
- electronic infection prevention and surveillance systems (back-end systems); and
- e-prescribing and electronic health record systems.
Hospitals that may be considering implementing a CDSS will need to consider the status of the existing infrastructure, including legacy patient information systems, the quality and size of servers, access to a sufficient number of computer terminals for staff, and the potential costs.
Integration of CDSS with existing systems usually requires customisation and modification to ensure interoperability. Other systems can be implemented as standalone systems.
Hospitals may use more than one of these systems to support the AMS program.
Mobile applications
With increasing smartphone use among clinicians, clinical portals and resources, including those containing guidelines, are becoming increasingly mobile device-compatible. This helps to make passive decision support accessible at the point of care, thus potentially increasing the frequency of its use. Smart phone applications can assist with:
- dissemination of disease- or antimicrobial-based guidelines;
- calculators for dosing;
- access to antibiograms; and
- access to policies and procedures relating to antimicrobial use.
While smartphone applications facilitate rapid dissemination of content and is particularly useful for hospitals with poor IT infrastructure, there are a number of risks associated with their use. Version control may be a potential concern with smartphone applications, as users are prompted to install updates at their discretion. Third-party applications may not support or be compliant with local practices and clinical guidelines, although the option of local customisation may be offered by some applications.
The efficacy of these apps to improve antimicrobial prescribing quality is uncertain as the use of these apps by junior staff may not influence the prescribing of senior clinicians.
Electronic antimicrobial approval systems
Approval systems have been implemented at various hospitals globally and their impact on antimicrobial prescribing has been researched widely. They have been found to be effective in reducing consumption of targeted antimicrobials as well as costs.
Antimicrobial restriction and approval systems may be phone-, paper- or computer-based, and can constitute a core element of AMS programs. Approval systems may have pre-prescription or post-prescription authorisation mechanisms. They may include clinical decision support through algorithms or alerts for particular conditions such as allergies. Authorisation and approval systems can be implemented in the absence of an electronic health record (EHR) or electronic medication management (EMM) system and can function well with minimal integration.
These systems support the AMS service through:
- enforcement of the antimicrobial formulary (including the ‘traffic light’ approach for unrestricted, restricted and highly restricted drugs);
- enforcement of approved indications by drug;
- provision of educational opportunities for the prescriber;
- facilitation of the post-prescription review process by the AMS team; and
- facilitation of reports and feedback using approvals data.
Local customisation of content is an essential process that needs to be undertaken prior to implementation. This includes reviewing an approved antimicrobial formulary, lists of indications, and any additional prescribing rules (e.g., the requirement for a chest X-ray that confirms pneumonia prior to an approval for ceftriaxone in community-acquired pneumonia).
Approval systems can further improve the appropriateness and quality of prescribing by directing the attention of the AMS team to prescriptions that require review. Therefore, these systems are best combined with an antimicrobial team to review patients at some time post-approval, e.g., 24-48 hours.
These systems can be implemented as part of a mandatory process so that an antibiotic is not administered to the patient without an approval, or they may be implemented as part of a voluntary process. In the either case, adherence needs to be continually encouraged and monitored as systems that require the prescriber to select indications can be ‘gamed’ through the erroneous selection of indications.
Infection prevention and surveillance systems
Third-party infection prevention and surveillance systems are commonly used to monitor hospital acquired infections (e.g., catheter-related bloodstream infection, C. difficile) and antimicrobial resistant isolates (e.g., methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci), and facilitate surveillance activities (including the reporting of antibiograms). They can integrate data from different sources, including pharmacy dispensing, laboratory, diagnostic imaging and EHR systems. This data integration takes place at the ‘back end’, generating alerts when customised criteria are met, and prompting the infection prevention team to act on these alerts.
Many of these functionalities support the AMS service (which should be working in conjunction with the IPC service). In advanced systems that integrate microbiology results and pharmacy/medication management data, examples of clinical interventions could include interventions for bug-drug mismatches (e.g., known resistance to a prescribed drug), redundant anaerobic coverage, and positive blood cultures. These systems use algorithms and rules-based criteria to generate alerts.
The suitability of infection prevention and surveillance systems can be assessed based on local infrastructure needs. Infrastructural limitations, such as lack of interoperability between legacy pathology and EMM systems, and other issues, may make it difficult for facilities to adopt third-party systems. The clinical workforce also needs to be sufficiently large to monitor and act on alerts as well as to undertake reporting.
Implementation reports suggest that these systems may generate large volumes of alerts, of which only a proportion may warrant interventions. Continuous management of large volumes of superfluous alerts can result in fatigue. Therefore, the alerts criteria would need to be carefully managed and refined during implementation, and facilities would need to carefully assess their infrastructure and workforce capacity, as well as the amenability of their legacy pathology and pharmacy dispensing systems.
Electronic prescribing and medication management systems
Uptake of electronic medication management (EMM) systems varies around the world. In the US and UK, EMM systems have been implemented in a large proportion of hospitals, whereas uptake may be lower (although increasing) in other regions and low- and middle- income countries. Functionality and sophistication can vary across systems. EMM systems may enable computerised entry of physician orders (e-prescribing), medication review, dispensing, monitoring of drug administration, and (potentially) decision support.
Studies evaluating EMM systems have found that systems with decision support functionality can lead to reductions in adverse drug events, readmissions and healthcare costs. EMM systems providing ‘front-end’ decision support are effective in improving the quality of prescriptions and the treatment record, and reducing transcription errors. They may be less effective in identifying the incorrect selection of drugs, addressing which would require post-prescription review (PPR).
It is important to recognise that while EMM systems reduce transcription errors associated with paper-based prescribing, there are other potential opportunities for harm.
Some concerns about EMM systems have been well-documented: large numbers of drug and dosing combinations; erroneous auto-complete directions that contradict intended physician orders; untimely transmission of discontinuation orders; inconsistent decision support mechanisms (potentially resulting in high order-override rates); and lack of support for local customisation. Alert fatigue is a major problem, with some reports citing that >90% of alerts are overridden or ignored by clinicians.
There are several opportunities to support AMS, including through:
- configuration of default values, doses, frequencies and routes of administration;
- configuration of drug interaction and allergy alerts;
- enforcement of formulary restriction;
- configuration of order sets for disease conditions (e.g., pneumonia, sepsis);
- configuration of rule-based triggers for AMS review (e.g., IV-to-oral switch);
- documentation of interventions within the EHR; and
- data extraction to support reporting.
The AMS program should be involved with EMM implementation. Customisation of local drug databases, rules, and order sets should be overseen by the AMS team. This will often require significant resources from clinicians and IT services and investment by the institution. Typically, these systems are less responsive to change.
Advanced decision support systems
Advanced decision support systems use machine learning and case-based probabilities to provide patient-specific recommendations. These systems may be able to identify potential infections, pathogens and treatment options based on input about patient signs, symptoms and test results (e.g., sepsis).
Although these are still in early stages of conception and development, and are often ‘home-grown’ within an institution, a few have been successfully trialled and evaluated for their impact on antimicrobial prescribing and patient outcomes (e.g., bacterial sepsis).
Some of the techniques used in these systems include:
- causal probabilistic network approach to identification of pathogens, by specimen type or underlying conditions of patient;
- case-based probability; and
- machine learning algorithms.
The role of these systems in hospitals with few resources and old legacy IT infrastructure is likely to be very limited in the short term.
Implementation of information technology systems to support antimicrobial stewardship
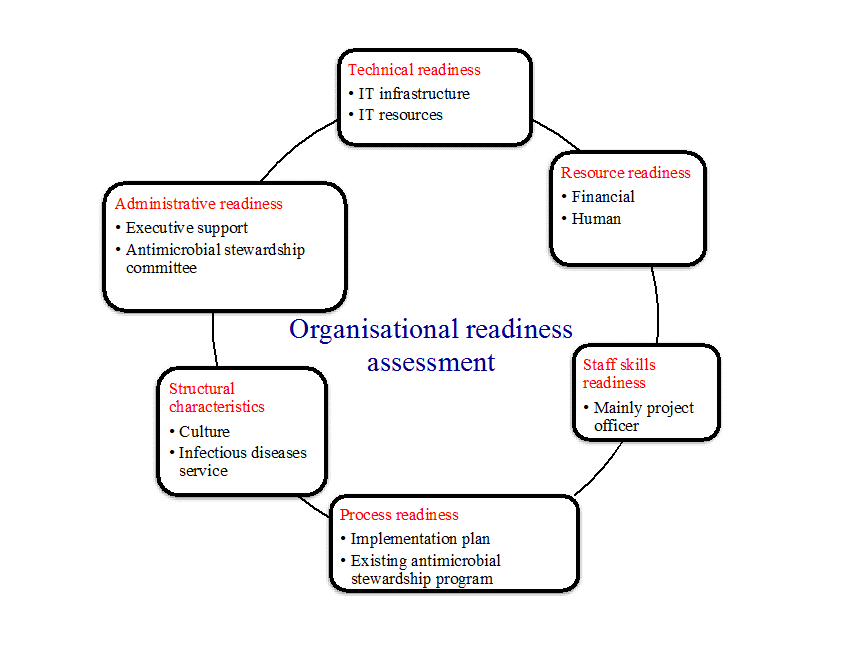
Several important factors can influence the implementation of a new technology within the busy hospital clinical workflow, including:
- executive support and investment into the system;
- the existence of an established AMS program that can oversee the implementation;
- appropriate local IT technology support and resources (such as server or computer terminals);
- adequate project support during the preparation and implementation phases; and
- clinical readiness for the adoption of the new technology.
It is imperative that clinicians and AMS team members are comprehensively involved in the planning and decision-making around the implementation of the system. Clinicians and the AMS team need to be involved in every stage of the process – from scoping and developing functional specifications through to the final implementation. Local customisation is critical, and each hospital unit and speciality would need to have input into formulary restrictions, antimicrobial indications and rules, for example.
Several factors would guide the choice of an IT system to support the AMS program.
- Technical readiness (strong IT infrastructure and dedicated IT resources) is a necessity, and local technical capacity should guide the selection of a system ideally suited to local needs.
- Cost is an important consideration and can be a barrier, so ideally a system that serves local needs and supports practicable AMS strategies should be selected.
- AMS teams should consider the volume of data that they can reasonably manage and use this to guide their selection and implementation of a CDSS.
Measuring user acceptance is necessary, and training would need to be provided throughout the deployment and beyond. Clinician uptake will be influenced by several factors, including speed, usability, integration into the workflow, simplicity, and availability of training and evidence of impact.