Antimicrobial Stewardship
Governance and policy
- The success of an AMS program is dependent on the leadership and support of hospital management and senior medical staff.
- Hospital management is responsible for ensuring that an AMS program is developed, implemented, evaluated and adequately funded.
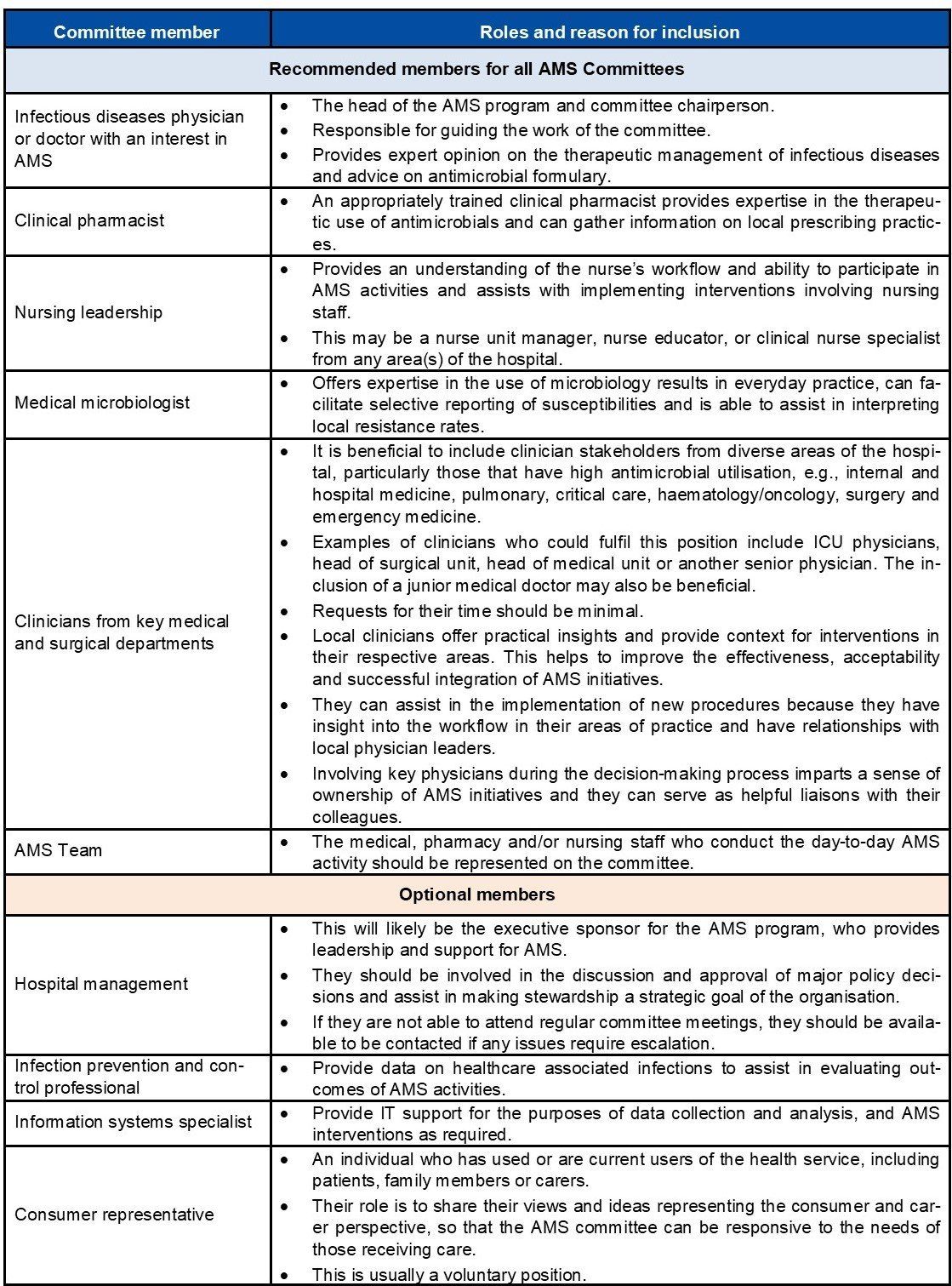
- An AMS committee (or equivalent) should be established to oversee and provide strategic direction for the AMS program.
- The committee should consist of a lead doctor, clinical pharmacist, nurse, microbiologist and clinicians from key medical and surgical departments.
- The committee should have a documented terms of reference.
- The roles of the committee include:
- Developing and maintaining antimicrobial policies, procedures, clinical pathways, formulary restriction and approval processes.
- Overseeing interventions and educational strategies for clinical staff.
- Ensuring appropriate monitoring, reporting and feedback of AMS processes and indicators to relevant hospital staff.
- All hospitals should have an antimicrobial prescribing policy and procedure to promote rational antimicrobial prescribing practices.
- making AMS a goal of the organisation;
- dedicating sufficient funding and resources for AMS-related activities;
- allocating an executive sponsor;
- communicating to staff the need for change;
- scheduling time to review progress and provide advice;
- supporting training and continuous education;
- ensuring accountability from all levels and across relevant clinical departments through continuous monitoring of performance; and
- building an enabling environment to support AMS-related activities, e.g., IT systems to monitor antibiotic use.
- leading by example and striving to deliver evidence-based care in everyday practice;
- participating in AMS activities such as guideline development and review;
- attending education sessions;
- reflecting on results of audits and participating in discussions; and
- promoting a culture of learning, transparency and quality improvement.
In addition to the above, maintaining active links to other relevant groups contributes to current, well-informed discussions, encourages resource sharing and may foster integrated approaches to common problems within or across facilities. Members of these other related groups and committees may also form part of the AMS committee.
Some highly recommended committees that can be involved include infection prevention and control, drug and therapeutics, medication safety or other quality committees.
Responsibilities of the antimicrobial stewardship committee
The roles and responsibilities of the AMS committee include:
- Develop and maintain antimicrobial policies, procedures and clinical pathways for antimicrobial treatment and prophylaxis.
- Define and maintain a formulary restriction and approval process that includes restricting broad-spectrum antimicrobials to patients in whom their use is clinically justified.
- Develop and implement interventions and educational strategies for medical officers, nurses, pharmacists and other clinical employees on appropriate antimicrobial prescribing and AMS principles, and monitor outcomes.
- Maintain an awareness of local antimicrobial resistance patterns amongst local pathogens, and relevant local outbreaks of infection and to consider whether these may need to influence antimicrobial prescribing guidelines.
- Monitor antimicrobial usage, including appropriateness of prescribing relative to evidence-based recommendations.
- Participate in activities that permit benchmarking and comparisons of prescribing between facilities where appropriate and provide guidance on proper interpretation of these findings.
- Ensure antimicrobial stewardship process and outcome indicators are measured and reported to the hospital management and relevant committees.
- Ensure that there is feedback of clinically relevant data regarding prescribing behaviours to prescribers and to other stakeholders, e.g., nurses and pharmacists in a way that they can understand.
- Ensure that AMS activities are aligned with other hospital activities.
- Regular meetings should be established with defined objectives, action plans and measurement of progress and outcomes. AMS committees will often meet monthly, or, for smaller centres, second or third monthly. Any less often than quarterly (3 monthly) is rarely acceptable but might be relevant for a small centre with a limited spectrum of diagnoses managed (e.g., a day-procedure hospital).
Creating a terms of reference
The AMS committee should have documented terms of reference that are readily available within the organisation, and include but not be limited to:
- objectives;
- roles and responsibilities;
- membership: positions of members, details of which members are the chair and secretary;
- meetings: minimum number per year and quorum requirement for decisions to be passed;
- reporting structure to hospital management and relevant committees; and
- committee planning and evaluation, annually.
A sample terms of reference is included at the end of this page.
The antimicrobial prescribing and management policy
All hospitals need an antimicrobial prescribing and management policy.
The AMS committee is responsible for the development, implementation and revision of this policy with the support and commitment of the hospital administration.
The policy should be simple, clear, clinically relevant, and readily accessible in a user-friendly form. It should have an expiry date and be regularly reviewed.
The development of a policy for AMS is a fundamental step towards empowering and consolidating an AMS program. An effective AMS policy will communicate key concepts to relevant staff members and also establish AMS as a quality improvement priority and validate the authority of the AMS team.
The policy gives the AMS committee its mandate to act. It makes clear what the overarching objectives of the committee are, and what level of oversight the committee has regarding the prescribing practices of staff. The policy also describes the expectations of prescribers. Generally, this will include that prescribing practice is expected to be evidence-based where possible and variations should be clinically justifiable.
At a minimum, the policy should include:
- A statement of intent that the organisation strives to ensure prudent antimicrobial use to deliver high quality and safe patient care.
- That the organisation delegates the AMS committee to provide recommendations to support AMS.
- That the organisation expects all prescribers to prescribe according to available national prescribing guidelines. Any local variations in prescribing guidelines should be endorsed formally by the AMS committee. Where variation is required for individual patients, it should be clinically justifiable.
- That the organisation expects prescribers to prescribe according to the endorsed formulary. This includes restrictions on access to last-line, broad-spectrum drugs for which a local procedure should be followed to ensure that appropriate expert advice is sought.
- That prescribers understand that monitoring of prescribing practices will be undertaken to enable analysis, reflection and promote ongoing improvement in care.
The policy will generally be accompanied by a procedure that provides more detail. This will include the actual formulary and restricted antimicrobial drug list, the expected procedure to seek approvals pre-prescription, and the expected manner of obtaining post-prescription review for individual patients and prescriptions. The procedure is typically updated more frequently than the policy.
A sample AMS policy is included here.